Calculation of Dilution Made Practical for Real Laboratory Work
Table of Contents
- Why Calculation of Dilution Matters
- The Core Idea Behind Dilution
- Understanding the Equation
- How to Calculate Step by Step
- Why Unit Consistency Is Critical
- Using a Dilution Calculator
- Serial Dilution Explained
- Calculation in Molarity Work
- Stock to Working Solutions
- Common Mistakes
- Research Based FAQs
Calculation of dilution is one of those skills that looks simple on paper but causes real problems in practice when it is misunderstood or rushed. Anyone who has spent time in a laboratory knows this. A small error in dilution can ruin an experiment, waste expensive reagents, or produce results that look correct but are scientifically meaningless.
Whether you are preparing a buffer solution, diluting a drug stock, working with enzymes, or performing serial dilutions for microbiology or cell culture, dilution is not just math. It is a practical laboratory process that connects concentration, volume, accuracy, and technique.
This guide is written for people who actually prepare solutions, not just students memorizing formulas. It explains calculation of dilution in a way that matches how dilution is done in real labs, why mistakes happen, and how to avoid them using a reliable Dilution Calculator.

Why Calculation of Dilution Matters More Than People Realize
In theory, dilution is straightforward. In practice, it is one of the most common sources of error in laboratory work.
A solution that is too concentrated may inhibit enzymes, kill cells, or cause precipitation. A solution that is too dilute may produce no measurable effect at all. In both cases, the experiment fails quietly. You might not even realize the dilution was wrong until days later.
Calculation of dilution matters because it directly controls experimental conditions. When concentration changes, reaction rates change. When volume changes, dose changes. When both are wrong, the data cannot be trusted. For more on the importance of concentration in reactions, you can refer to this guide on solution properties.
This is why experienced researchers rely on a dilution calculator rather than mental math, especially when switching between units like M, mM, µM, mg per mL, or percentages.
The Core Idea Behind Calculation of Dilution
At its heart, dilution is about conservation. The amount of solute does not change, only the volume does.
That idea leads to the most important relationship in dilution chemistry, often called the dilution equation.
C₁ = Initial Concentration (Stock)
V₁ = Initial Volume (Used)
C₂ = Final Concentration (Desired)
V₂ = Final Total Volume
This equation is the foundation of calculation of dilution. It appears simple, but each term has physical meaning. Everything you do in dilution chemistry is some rearrangement of this equation.
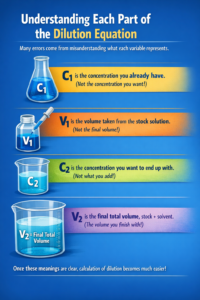
Understanding Each Part of the Dilution Equation
Many errors come from misunderstanding what each variable represents.
- C1 is not the concentration you want. It is the concentration you already have. This sounds obvious, but it is one of the most common mistakes beginners make.
- V1 is not the final volume. It is only the volume taken from the stock solution.
- C2 is the concentration you want to end up with, not what you add.
- V2 is the final total volume, including both stock and solvent.
Once these meanings are clear, calculation of dilution becomes much easier.
How to Calculate Dilution Step by Step
Let us walk through how to calculate dilution in a realistic laboratory example.
Imagine you have a 5 M stock solution of NaCl. You need 200 mL of a 1 M solution.
Calculation Example
Knowns: C1 = 5 M, C2 = 1 M, V2 = 200 mL
Find: V1
Formula: V1 = (C2 × V2) / C1
Result: V1 = (1 × 200) / 5 = 40 mL
This means you take 40 mL of the 5 M stock solution and add solvent until the total volume reaches 200 mL. The remaining 160 mL is solvent.
Why Unit Consistency Is Critical in Dilution Calculations
One of the fastest ways to make a dilution error is mixing units. If concentration is in mM and volume is in liters, the math may still work numerically, but the result may not make physical sense.
A proper dilution calculator automatically handles unit conversions. It allows you to enter concentrations in M, mM, or µM and volumes in L, mL, or µL without forcing you to convert manually. This matters especially when working with very small volumes or very low concentrations.
Using a Dilution Calculator Instead of Manual Math
In modern laboratories, manual calculation of dilution is rarely the best approach. A good dilution calculator does more than solve the equation. It guides the user, checks for impossible values, and presents results in a form that can be directly followed at the bench.
For example, if someone accidentally enters a final concentration higher than the stock concentration, a proper tool will flag this as physically impossible rather than producing a misleading number. That is why calculation of dilution should be supported by a dedicated dilution calculator rather than done on a calculator app or scrap paper.

Serial Dilution and Why It Is Different
Serial dilution is a special case of calculation of dilution used when extremely low concentrations are required. Instead of diluting directly from stock to final concentration, the solution is diluted step by step. This approach reduces pipetting errors and improves accuracy. You can read more about standard serial dilution protocols here.
For example, if you need a 1 µM solution from a 1 M stock, a single step dilution would require pipetting 1 µL into 1 L. This is impractical and inaccurate. Instead, you might perform a series of 1:10 dilutions. A good dilution calculator with serial dilution support will automatically generate each step, show the concentration at every stage, and tell you how much to transfer and how much solvent to add.
Calculation of Dilution in Molarity Based Work
In chemistry and biology, molarity is one of the most common ways to express concentration. Calculation of dilution involving molarity often appears in two forms.
- The first is dilution from one molarity to another using the dilution equation.
- The second is calculating molarity from mass and volume.
This is where a molarity calculator becomes important. The molarity formula is M = moles / volume. Since moles depend on molecular weight, accurate molarity calculation requires correct molecular weight input. When molarity calculations and dilution calculations are combined in one tool, it becomes much easier to move from solid preparation to solution dilution without errors.
Calculation of Dilution from Stock to Working Solutions
In pharmaceutical and biochemical labs, stock solutions are often highly concentrated for storage stability. Working solutions are prepared fresh by dilution. For example, a drug may be stored as a 10 mM stock and used at 10 µM in experiments.
Calculation of dilution in this case must be precise, because dosing errors can invalidate entire studies. A dilution calculator designed for stock to working solutions helps by clearly separating stock concentration, working concentration, stock volume used, and final volume. It also reduces mental load, allowing researchers to focus on experimental design rather than arithmetic.

Common Mistakes in Calculation of Dilution
Even experienced lab workers make dilution mistakes. Here are common ones:
- Forgetting final volume includes stock volume: V2 is the total, not just the added solvent.
- Confusing dilution ratio with dilution factor: A 1:10 dilution means one part stock and nine parts solvent, not ten parts solvent.
- Ignoring pipette limits: Trying to pipette 0.2 µL accurately is unrealistic, even if the math is correct.
A proper dilution calculator highlights these issues and suggests better approaches, such as intermediate dilutions.
Research Based FAQs
The dilution equation assumes conservation of solute. For most aqueous solutions at moderate concentrations, volume changes are negligible, making the equation reliable.
Serial dilution is preferred when the required dilution factor is very large or when pipetting very small volumes would introduce error.
Yes. Temperature can affect solution volume and solubility. High precision work should be performed at controlled temperatures.
Molecular weight determines how mass converts to moles. Incorrect molecular weight leads directly to incorrect concentration.
Because calculators reduce human error, enforce physical constraints, and improve reproducibility.
The equation still applies, but acids require special handling due to heat generation and volume contraction.
Changing concentration alters reaction rates according to rate laws, which is why precise dilution is critical.
No. They support trained users, but understanding chemistry and technique remains essential.
Ready to Simplify Your Lab Work?
Use our professional, accurate, and free Dilution Calculator today to ensure your experiments are precise every time.
Start Calculating Now